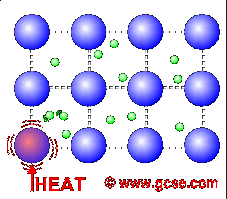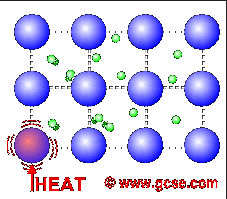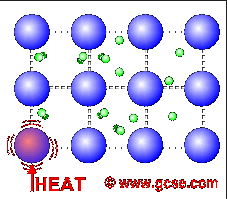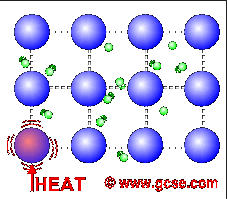
All metals are good conductors of electricity. For a similar reason, they are also good conductors of heat.
In metals, not only do the atoms vibrate more when heated, but the free electrons charge around more as well. These transfer the energy much faster than just vibrations in bonds.
|